At this moment we have a sufficient number of registrations to guarantee the number of participants (200). Therefore, the period of registration has ended. Should you want more information regarding the study, this is to be found at the page ‘Information for the particpant’.
Information for the participant
On this page you can find more information about the study, for the main part it is a copy of the study’s informed consent form. To be able to participate you will be asked to sign this form at the study site, which means that you confirm that you understand the following aspects of the study and that you agree with them.
Purpose and description of the study
This is the first study in Belgium that examines how men who have sex with men (MSM) use PrEP and whether they find it acceptable.
More specifically, we will evaluate whether, in real life, PrEP is an acceptable and useful additional prevention tool for HIV among MSM in Belgium. We will also gather more information regarding MSM’s current preventive needs, whether taking PrEP changes how MSM use other preventive strategies, and whether it influences the risk to get infected with other sexually transmitted infections (STIs).
If you decide to participate in the study, you will be able to choose your own regimen: daily use (= comparable to the birth control pill for women, meaning you take a tablet each day at the same time) or event-driven use (= with intervals, which requires that you know in advance when you will have sex). You will be able to change your regimen or to discontinue the use of PrEP if you prefer to do so, however this is best discussed with a study physician during a study visit. When you discontinued using PrEP, you will be able to restart the next study visit.
The study will be carried out at the HIV/STI Clinic of the Institute of Tropical Medicine. Two hundred men will be included and the study’s duration will be 18 months for every participant. This means that every participant will be able to receive PrEP and and to be followed up medically and psychosocially for 18 months.
Study visits
If you decide to participate we will ask you to come to the clinic for a screening visit. During this visit we will examine whether you are eligible to participate. When you decide to participate we ask you to come for study visits at regular intervals.The first two study visits (screening & enrolment) can take up to an hour and half, whereas the follow-up visits will be shorter in time. They will mostly be planned during an afternoon, although this might change in the future. After the screening visit the following visits are planned: a next visit is planned 1-2 weeks later, followed by a follow-up visit 1 month, one after 3 months and then every 3 months untill you reach 18 months. In total, there will be 9 visits. It is always possible to contact the study staff to plan an additional visit in-between if needed (this is called an unscheduled visit).
Study visits can start at the following moments:
- Tuesday: 14.30h – 17.30h
- Wednesday: 14.00h – 16.00h
How the study is done
During every study visit you will be examined and you will be able to talk to the study personnel about your health and sexual behaviour in an open manner. He or she can help you with questions you may have about the study, your health, and a correct use of the medication.
We will not judge your sexual behaviour during the study, however we are very interested in this behaviour from a scientific point of view. Moreover, all information will be confidentially handled. Therefore, we would like to ask you to be as honest as possible when answering all questions, they can be very personal or sensitive.
The physician and/or nurse will also be able to help you to cope with possible negative reactions you may get from people around you.
Screening visit (start of the study)
If you decide to participate in this study and if you are eligible to participate, you will – after signing the informed consent – be examined by a study physician. We will also ask you to provide blood (a maximum of 20 mL or 2 blood tubes), urine, a rectal- and a throat swab. On those samples we will carry out an HIV test, liver- and kindney tests. These tests will determine whether or not you will be able to participate. You will be asked to come back for your result 1-2 weeks later at the enrollment visit. For none of the physical examinations fasting is required. Furthermore, we will only carry out tests that concern this study.
We advise you to be extra cautious during the period between the screening and enrolment visit, and to reduce sex with a high risk of HIV to a minimum.
Enrolment visit (Start PrEP use)
When the tests show that you can participate in the study and you still would like to participate you will be enrolled in the study. Then the following procedures will take place:
- Physical examination by the study physician
- The study physician will ask questions regarding your medical history, medication and recreational drug use
- You will be asked which PrEP regimen you prefer:
- Daily PrEP use
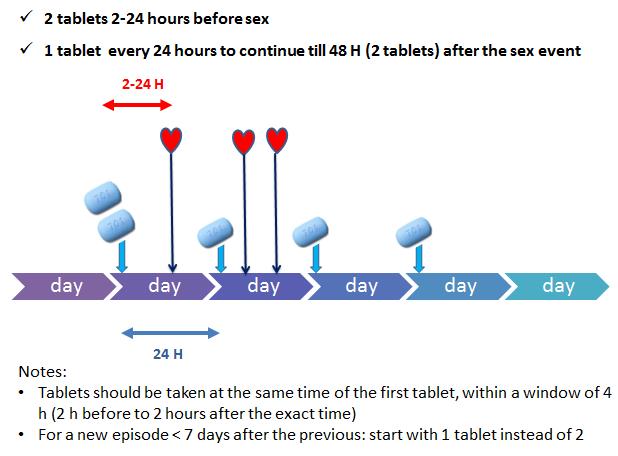
- Event-driven PrEP use: Two tablets 2-24 hours before an eventual sex act, one tablet every 24 hours later during the sexual active period and one tablet for two days after the sexual active period. Below you see a schedule that explains how to use event-driven PrEP use correctly:
- You will receive adherence counseling, safer sex counseling and free condoms and lubrication.
- You will be asked to complete a diary during the whole study period (which can be completed online or on paper, depending on what is most convenient to you) for documenting your pill use, when you had sex and whether you used condoms. You will be instructed to complete this diary daily and as accurate as possible. This is important for the study to achieve trustworthy results.
- You will be asked to complete a questionnaire on a tablet computer that includes questions on your general well-being and sexual behaviour. If you don’t feel comfortable to use a tablet or if a problem occurs, you can always ask assistance from the study staff.
- You will receive the medication at the HIV/STI Clinic. You have to bring back the package, the bottle and the remains of your medication at the next study visit!
- A next follow up visit will be scheduled approximately 4 weeks later.
- In case the physician finds it necessary, additional blood (max 7.5 mL) can be taken to confirm your HIV negative status.
Follow-up visit Month 1
During this follow up visit, we will collect some information on side effects or illnesses that you may have encountered. We will also ask more information about your medication use since the previous weeks. The following procedures will also be performed:
- A physical examination by the study physician
- Blood (two tubes of 7.5 mL) will be collected for HIV and drug level testing
- We will ask you if you agree to have additional testing performed to assess the levels of the PrEP drugs in your body. To do this, we would like you to provide approximately 100 strands of hair (this is the thickness of a pencil which will be cut at the back of your hair). We will use the hair to perform drug level testing, which is another way to measure adherence to the medication. We will do this only if you provide your additional informed consent for hair collection.
- We will discuss the PrEP use very thoroughly and in case you would like to switch to another regimen or discontinue PrEP use this will be possible during every follow-up visit.
- Your diary information will be discussed with a social scientist (whether or not this is fully completed) and the correct pill taking will be discussed with the study physician. The purpose is to find solutions for potential problems with adherence and safer sex, not to judge your behaviour. Therefore, it is important that you report your experiences in the dairy accurately. If you feel that it is difficult to take the pills correctly, you can discuss the option to change the regimen for your own health with the physician.
- In case you would like to continue PrEP, you will receive the necessary amount of medication. You have to bring back the package, the bottle and the remains of your medication at the next study visit!
- In addition you will receive safer sex counseling and free condoms and lubrication
- You will be asked to complete a questionnaire on a tablet computer that includes questions on your general well-being and sexual behaviour. If you don’t feel comfortable to use a tablet or if a problem occurs, you can always ask assistance from the study staff.
- You will be invited to come back to the HIV/STI clinic for the next study visit after approximately 8 weeks.
Follow-up visit 3, 6, 9, 12, 15, 18
From now on, you will be invited to the clinic every three months.
The same procedures of month 1 will take place. In addition, we would like to ask you to provide more blood (in total this will be a maximum of 3 tubes per visit), one rectal swab, one throat swab and urine samples to check for STIs and to check whether the liver and kidneys are working well. As previously, you will be able to discontinue/restart or change your PrEP regimen at every follow-up visit.
The total duration of your participation into the study will be 18 months. It is possible that you would quit participation to the study earlier, because:
- You prefer to do so. You can quit participating at any moment, although we do encourage you to complete the study.
- You become HIV positive.
- The study physician decides this would be best for your own health.
Additional interviews
At the follow-up visits at months 1, 3, 6, 9, 12, 15 and 18, you may be invited to participate in an individual interview. This will be to learn more about your personal experiences with and opinions about PrEP. If you decide to participate in such an interview, the procedures will first be explained to you. You will be given a separate consent form to sign. Participating in such an interview is completely voluntary. If you decide you do not want to participate, you will still be able to participate in the study.
Is there any chance that I do become HIV-postive?
Research has shown that PrEP reduces the risk of HIV-infection, and thus that PrEP is effective among MSM. Different studies have shown that the risk of HIV-infection was lower among those men who had taken their pills correctly (up to 92% less) compared to those who did not take PrEP. Using condoms always and correctly will provide you the best protection against HIV and other STIs (against which PrEP has no effect). Whether or not you choose to use condoms is your own personal decision. Your study counselor can help you in this.
There is always a chance – however small and depending on your own behaviour – that you would acquire HIV. However, that risk is reduced when you take the medication correctly, meaning that you would not miss any dose.
What happens in case you become HIV infected during the study?
We will monitor your HIV status at every visit except at the enrollment visit. In case of the very rare event that you should become HIV infected during the study, you can no longer continue, but all necessary steps will be taken to refer you to the best HIV care available, and to immediate psychosocial counselling.
Should this happen to you, however small the chances, you will have a lot of questions. More information can also be found on this website.
In the event that you would become HIV-positive during the study, you will no longer be able to participate in the study. For the study we will however still perform all procedures that are required at the final visit. In addition, the following procedures can take place:
- One additional blood sample of 7.5 mL will be taken for viral load testing and resistance testing.
- You may be invited to participate in an individual interview.
- You will be discontinued from the study but we will ask you if you agree that we can follow you up for safety reasons.
- You will be directed to an Aids Reference Centre of your choice for future care. The Institute of Tropical Medicine is such a center, where you can get medical and psychosocial care.
What do we expect from you in case you decide to participate?
In case you decide to participate, we will expect the following from you:
- Come to each scheduled visit. Please, take notice of the hours at which a visit can be scheduled. If you think you will be unable to keep to your scheduled visits, please do not consider to take part. However, we will do our best to be flexible in setting up appointments considering your availability as much as we can. Every visit will take about an hour to an hour and a half. Should it happen that you can’t keep your appointment, please inform the study staff as soon as possible and contact them to plan an additional in-between visit. If you do not show up to a visit without prior notice, we will contact you to arrange a new appointment.
- Provide truthful information about your medical history and current conditions.
- Provide truthful information about your actual medication and recreational drug use.
- Provide truthful information about your sexual behaviour, condom use and PrEP use both in the self-administered questionnaires and to complete the diary.
- Tell the study physician and/or counselor about any problems you have during the study.
- Inform the study physician and/or counselor if you experience any problems during the course of the study.
- Do not give the medication to another person. Sharing PrEP with HIV positive people can result in unwanted interactions with other antiretroviral medication. This could result in their medication not being effective anymore. Also for people who assume to be HIV negative, it could be dangerous to take PrEP without medical control.
Risks and inconveniences
Participating in clinical studies takes a lot of effort for participants, because everything needs to be well examined, measured and documented. These procedures may be experienced by some as excessive. Next to the inconveniences that are due to the physical examinations (blood sampling, throat swabs, and such) there is also some kind of time-investment expected of study participants: study visits will take place every three months during an afternoon (take notice, during the first visits this will be more frequent) and may take about an hour up to an hour and a half. Also completing an online diary daily, as well as mailing urine samples might be experienced as burdensome by some participants.
You may also experience some discomfort as a result of the medication:
If you have Hepatitis B, the infection could become more active once you stop taking PrEP. Very rarely, Hepatitis B can lead to liver failure and death. That is why your Hepatitis B status will be checked at the start and at the end of the study.
You may experience some side effects that may be related to PrEP use. These are usually seen in the first few weeks and may include:
- Nausea, diarrhea or vomiting
- Gas or bloating of the stomach
- Skin rash
- Headache
There may be rare but serious side effects. These include:
- Problems with the liver, although this is very uncommon
- Your kidneys may not function properly anymore
- A decrease in the density of your bones
- Allergies to the drug
Drug resistance
There is a risk that Truvada (PrEP) may cause resistance to Tenofovir or Emtricitabine (the antiretroviral medication where Truvada consists of) if you become HIV positive during the study. Resistance means that Truvada would not be effective against HIV anymore.
Stigma
Other studies have shown that you may experience negative reactions or stigma as a result of participating in a study concerning PrEP. This is most likely the result of insufficient knowledge or information about PrEP. This may prevent you for speaking freely about PrEP with friend, family or partner(s).
It is very important that you tell the study staff if you experience any side effects.
Benefits of participating
The advantage of participating is in receiving a prevention drug against HIV that is effective, in a controlled study, with all necessary medical and psychosocial counseling.
Truvada (PrEP) has been shown to be effective against HIV acquisition in men with sexual risk behaviour when taken correctly, and will be offered for free for a maximum of 18 months.
Other advantages are that you will be examined at every visit and you can talk to the study staff about your health and your sexual behaviour in an open way. He or she may help you with questions you might have about the study, your health, and your adherence.
You will not have to pay for the study visits nor for any of the tests.
Through your participation you will help to develop better HIV prevention activities for MSM in Belgium. Therefore, the gay community and men having sex with men at large may benefit from the study findings.
Compensation
All examinations related to you taking part in the study are free of charge to you. This is also the case for all examinations done during any unscheduled visit during the study.
You will not receive any additional compensation for your participation to the study. We also do not pay for travel costs or other expenses related to visits to the clinic.
Protection of your privacy
We will do everything we can to protect your privacy. The information you share with us will be stored in an electronic database. This information will not be shared with anybody, and can only be accessed by the research team and may be written down in your medical record. This information may also be examined by the organizer of this study (Institute of Tropical Medicine) or by the Belgian regulatory bodies to check that the information is correct. Your identity remains hidden since your personal data will be given a unique code. Your name will not appear in any reports or publication resulting from this study.
In accordance with the Belgian privacy law relating to the protection of personal privacy, you have the right to access your personal data and have it corrected if needed.
What will happen to your samples?
As mentioned in the procedures section above, we will perform several tests:
- HIV tests at every visit except at enrollment
- STI tests at baseline and three monthly visits: syphilis, Hepatitis B, Hepatitis C, genital herpes, gonorrhea (ie. the clap) and other STIs such as Chlamydia, Trichomonas and Mycoplasma genitalium.
- Kidney test at baseline and three monthly follow up visits
- Liver tests at baseline and final visit
- Drug level tests at every follow up visit
We will not perform all tests immediately and we won’t provide you routinely with the results of all of these tests, but you will be informed if this is important for your health. If you are interested in any other results as well, you have the right to obtain them.
The following samples will be collected and stored: blood, urine, rectal swab, throat swab, oral fluid and hair. Samples will be stored using your study ID number and not your name.
You will be asked to consent for long-term storage of your samples. If you agree, your samples will be stored for a maximum of 20 years after the final study report has been issued. They will be used only for research related to HIV or other STIs and, after getting approval of an Ethics Committee. No tests will be done on your genes (DNA). If you do not agree, all your samples will be destroyed after the final study report has been issued.
The informed consent form includes a separate space for you to give us permission to store these samples.
Ethics Committee
Before the start, this study was reviewed and approved by the Ethics Committees of the Institute of Tropical Medicine and the University Hospital of Antwerp. The study was also approved by the Belgian Regulatory Authority. These Ethics Committees also perform ongoing reviews of the study to make sure it is carried out in the safest way possible.
Voluntary participation
Your participation in this study is entirely voluntary. It is your choice whether you want to take part in it or not. No matter what you decide, all the services you may receive at this clinic will remain the same. You also have the right to stop your participation in the study at any time, even after you have signed the Informed Consent Form. You do not have to give a reason in case you want to stop being part of the study, but we would appreciate it. In that way we can improve our services.
What if new information becomes available?
Sometimes during the course of a study, new information becomes available about the medicine that is studied. If this happens, we will tell you about it and discuss with you whether you want to continue in the study. If you decide to continue, you will be asked to sign a new version of the informed consent form.
What will happen to the results of the study?
At the end of the study, the results will be detailed in a report and published in scientific journals or presented at conference. We also plan several information severals to inform the community. You will not be able to be identified in any of these. We will be able to provide you with a summary of the results if you request them.
Registration for participation
At this moment we have a sufficient number of registrations to guarantee the number of participants (200). Therefore, the period of registration has ended.